Human Platelet Lysate for Cell Therapy
Becoming human: GMP-compliant supplements for cell therapy
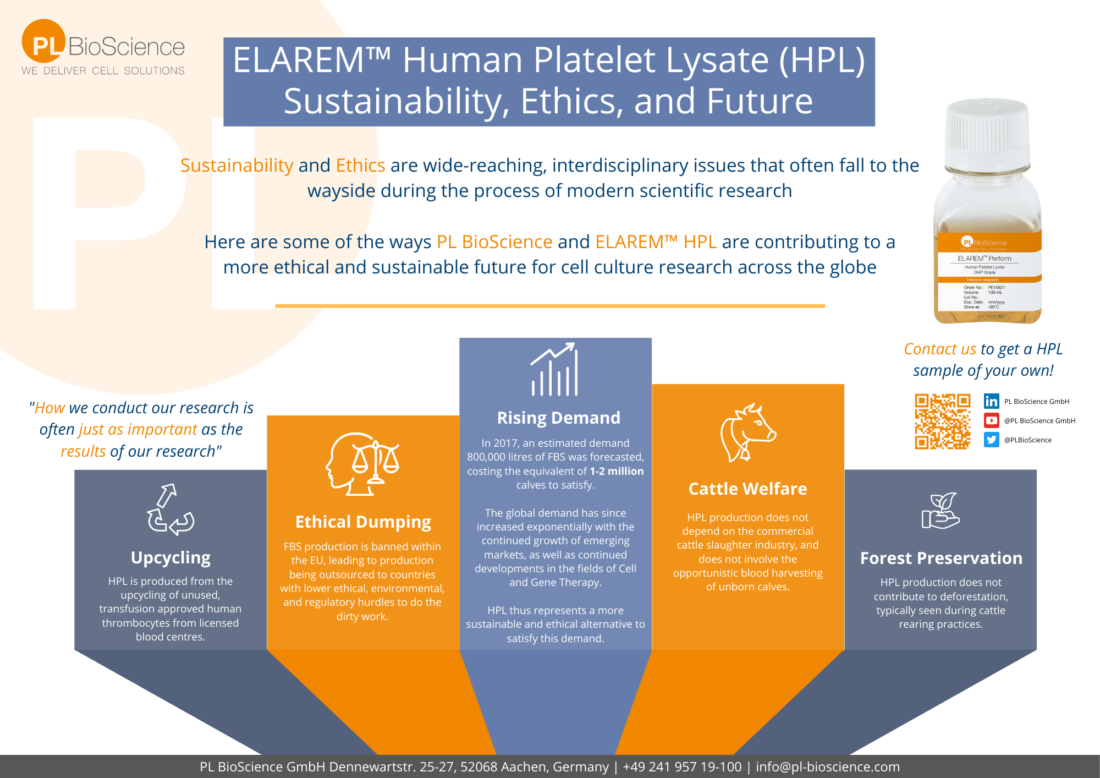
We were recently interviewed by European biotech media company Labiotech regarding our thoughts on cell culture reagent challenges, and our line of ELAREM™ Human Platelet Lysate (HPL) for cell therapy. The article features thoughts and quotes from CEO & Founder Hatim Hemeda, Field Application Scientist Silke Isenhardt, and Marketing Manager Nicholas Gouw. The article also describes how HPL can solve many of the problems facing clinical translation of cell therapy research when utilising the outdated standard of Fetal Bovine Serum (FBS).
Below is an excerpt from the interview article:
For the last decades, fetal bovine serum (FBS) has been the standard supplement in cell culture. However, FBS production processes are not thoroughly standardized, making them challenging to use under GMP conditions. In addition, FBS is associated with ethical concerns, as an estimated 2 million bovine fetuses are killed for FBS production each year. New developments include supplements prepared from human platelets – with the potential to overcome the limitations of FBS while enhancing cell growth and performance.
Read the full article on Labiotech’s website!
Written by Ute Boronowsky